
How India’s Covid-19 Vaccination Drive Will Work?
Yes, we are almost there! The nation is all set for the world’s largest COVID-19 vaccination drive. As final stage preparations are going on in every nook and corner of the country, let’s look into the details of India’s Covid vaccination plan.
India’s green light for the vaccination drive came on Sunday as the Drugs Controller General of India announced the emergency use approval for two vaccines – the Oxford University-AstraZeneca vaccine being manufactured in India by the Serum Institute of India (SII), and Bharat Biotech International Limited’s locally developed vaccine candidate, Covaxin.
Following this, the Union health ministry last week conducted a nationwide mock drill of the vaccination program at 285 session sites to test the end-to-end planned operations and the mechanism set up to ensure smooth vaccination for the highly infectious disease.
“The objective of the dry run is to assess operational feasibility in the use of Co-WIN application in a field environment, test the linkages between planning and implementation, and identify the challenges and guide the way forward prior to actual implementation. This is also expected to give confidence to programme managers at various levels,” the Union health ministry had said back then.
From Factory To Frontline
For executing the entire vaccination drive, the Centre will be utilising its new digital vaccine delivery management system – Co-WIN – that has been enhanced from its existing platform under the annual Universal Immunisation Programme. The entire process of vaccination drive can be further divided into three parts. Let’s look into it in detail.
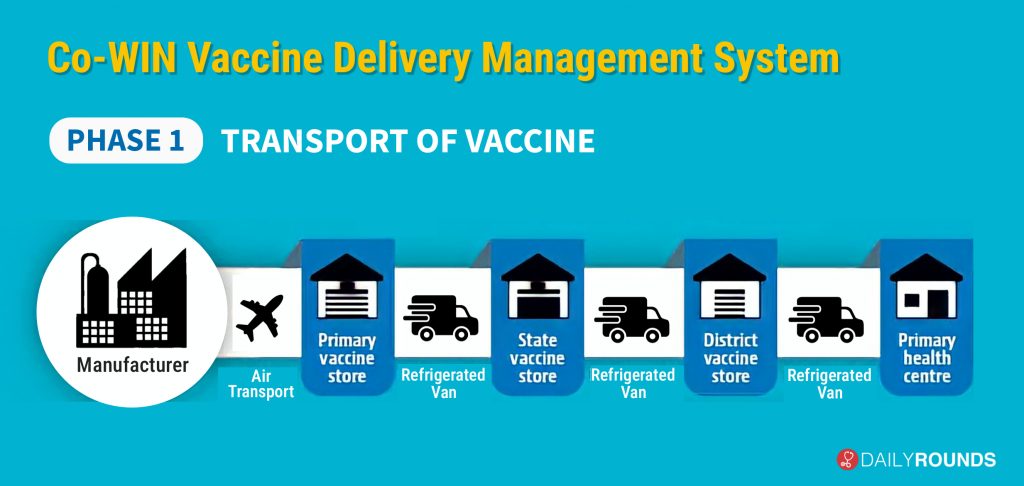
The “Co-WIN vaccine delivery management system” kicks off with the transport of the doses from the manufacturing plant. In the first step, manufacturers will transport the vaccines by air to primary vaccine stores run by the Union health ministry, which is called Government Medical Stores Depot (GMSD). Currently, there are four such depots in the country – one each in Karnal, Mumbai, Chennai, and Kolkata; from there it will be transported in bulk to the 37 State Vaccine Stores for further dissemination.
The vaccine will be transported under refrigerated condition and digitally tracked during the entire transit i.e. manufacturer to primary vaccine store to State vaccine store to District Vaccine store to Primary Health Centre. There will be temperature trackers inside all primary vaccine stores, State vaccine stores and District vaccine stores and information will be uploaded on a real-time basis about the temperature inside the facility onto a central server.
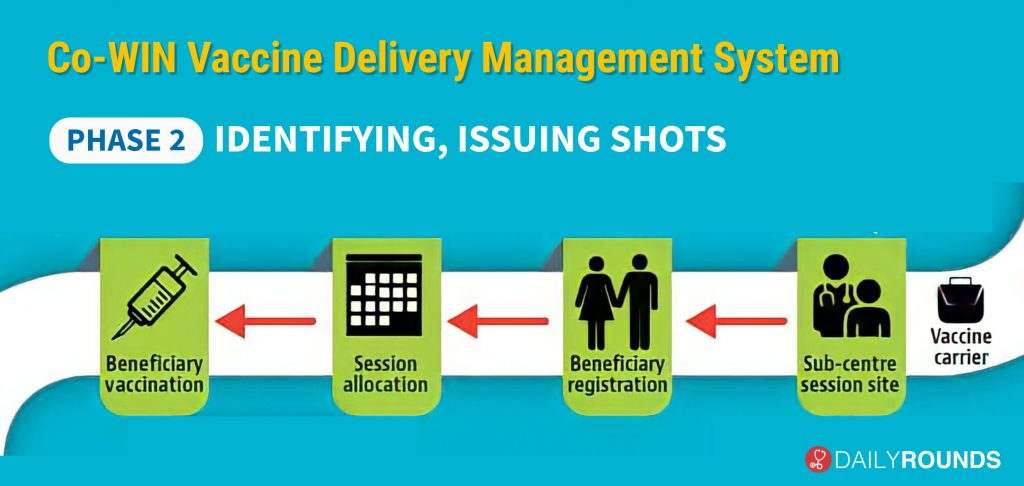
The second part of the drive will deal with the identification and registration of vaccine candidates as well as the administration of the shot. Once the vaccine reaches the sub-centre session site, there is a requirement for beneficiary registration, on the basis of which the District Magistrate can allocate the session based on the requirements.
However, there is no need for beneficiary registration of healthcare and frontline workers as their data will be taken from a bulk database that has been populated onto the CO-WIN Vaccine Delivery Management System.
At the same time, the risk population priority groups – people above 50 years of age, and those under 50 years with comorbidities – will be required to register on the app. While people above the age of 50 years will be identified based on electoral rolls, the data for those who are below the age of 50 with comorbidities will be compiled on the basis of an inclusive system that will grade diseases that may be more fatal with Covid. This system is being devised by an expert panel, which is expected to release the exact selection method in the coming days.
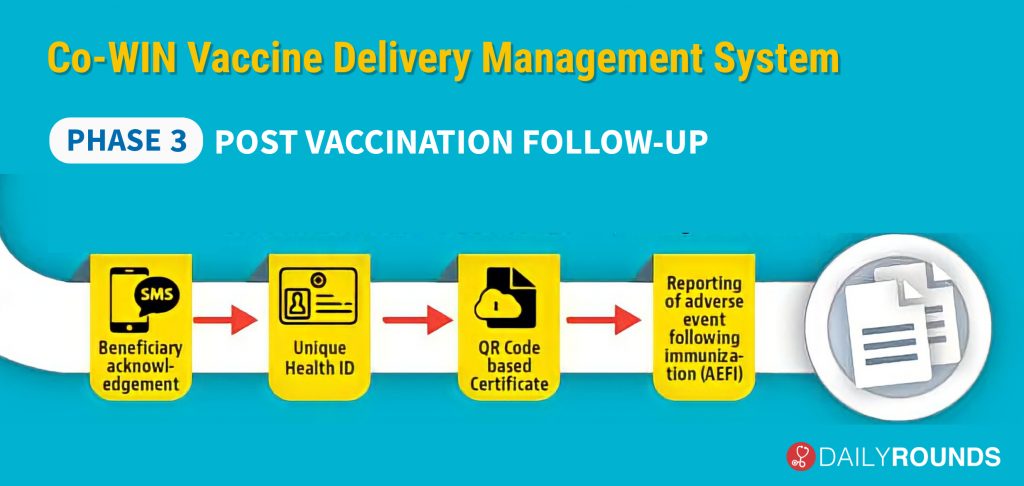
In the third, and final part of the process officials will be able to follow through with the beneficiaries after the immunisation. In case of any adverse effect following immunisation, there is a provision for real-time reporting. CO – WIN will also give permission to create a Unique Health ID. After both doses, a QR code certificate will also be generated which can be stored on the Government’s DigiLocker app.
Other features include SMS in 12 languages, 24X7 helpline, Chat Bot assistance, etc. As of now, more than 90,000 users have been trained in more than 700 districts. “No major issues were observed in the operational aspects of the program. Minor issues noted in Co-WIN for further enhancement have been addressed. All States expressed confidence in the operational guidelines and IT platform for large scale programme implementation,” said Union health secretary Rajesh Bhushan.
The Challenges In Front
India’s vaccine management has improved over the years thanks to a real-time supply chain management system known as the electronic vaccine intelligence network (eVIN). As of August 2020, it has been implemented in 32 states and union territories, and it will come in handy during the covid vaccination drive, that’s all set to begin.
Yet, the latest available audit of the vaccine chain conducted by the health ministry in 2017-18 shows that bottlenecks still exist. 26% of eVIN cold chain points reported instances of stock out during the period of assessment. More than a fifth of facilities reported wastage of vaccines.
India is ranked within the 51-75 percentile range among 89 countries on effective vaccine management as per a global analysis by WHO-UNICEF in 2018. Its performance was relatively poor when it came to following the required vaccine arrival procedures and using the MIS system for estimating the demand for vaccine, syringe, etc.
What adds to the vaccination challenge is the inter-state disparity in the distribution of cold chain points across the country. For instance, roughly 4 cold chain points serve 100,000 population in Gujarat, whereas there is just one cold chain for the same number of people in Jharkhand. The situation remains more or less the same in different parts of the country.
The unique requirements of the upcoming immunization drive pose yet another layer to the distribution challenge. Identifying beneficiaries, ensuring they show up on the day of vaccination, and administering a second dose after a month, and monitoring post-vaccination would require a lot of micro-level planning.
Also Read: How Close Are We To a COVID-19 Vaccine?
Based on the 2011 report under the immunization plan, only eleven states in India could reach 70% of the population, while 8 most populous states could reach under 50%. One of the major reasons for such low levels of penetration is primarily due to the unidentified bottlenecks in the system along with poor documentation of the process. This clearly shows that vaccinating the Indian population will not be that easy as it seems in the paper.
In addition to all this, the financial sustainability of the immunization plan also needs to be evaluated. Ideally, the national government should assume ownership and bear the cost of the immunization program, however, often the cost of the vaccine and operational cost can be quite overwhelming in a country as populous as India, leading to challenges in scaling up the program.
Yes, there’s indeed a huge list of challenges in front of us as we move closer and closer towards the Covid vaccine rollout. A few days from now, you might be standing in a queue, waiting for your turn to get the vaccine shot. For that to happen, there’s an urgent need to tackle the challenges and execute the plans in the best possible manner.
Follow and connect with us on Twitter | Facebook | Instagram