
Abbot Healthcare challenging state’s accusation that its cough syrup contains excessive codeine
The state governments of West Bengal and Himachal Pradesh have found a sample of drug maker Abbott Healthcare’s cough syrup Phensedyl to contain codeine above the allowed norm. Abbott healthcare is challenging this accusation. This case is important because the previously unreported case underlines the weakness of India’s unwieldy and poorly resourced drug and food regulatory system, the uncertainty it creates for foreign and domestic companies operating there and the potential risk to consumers.
Whether the sample of Abbott’s popular “Phensedyl” was a genuine product or a fake has not been established, but the suspect batch of 80,000 bottles has not been recalled.
In the latest dispute, the laboratory found that a sample of Phensedyl contained more than twice the labelled amount of codeine, according to several state drug officials and correspondence between regulators and Abbott seen by Reuters. Abbott has already received a ‘show cause notice’ from a drug regulator in Madhya Pradesh where Phensedyl is manufactured. If codeine levels are found to be excessive, it would violate India’s regulatory laws.
In response, Abbott has urged regulators not to take any action. The company also stated that it hadn’t found anything unuusal in its own samples and test results from third party testing of a sample from the same batch of 80,000 didn’t indicate codeine levels above the norm.
Its important to note that, previously Swiss manufacturer Nestle had got in trouble with regulatory authorities after they tested Maggi postive for lead. Later the charges were partially withdrawn, but the damage to reputation and market share because of such an allegation was heavy.
For Abbott, Phensedyl sales are estimated to be more than 3 percent of Abbott’s $1 billion India revenue. The sales are dwarfed by Abbott’s global annual sales of over $20 billion, but, as the Nestle case shows, fallout from safety scandals is unpredictable.
The Batch in Question
In November 2014, Phensedyl bottles were seized near the W.Bengal-Bangladesh border. Codeine based cough syrups are banned in Bangladesh and smuggling of this is high because it sells for more in Bangaladesh compared to India.According to a copy of the inspector’s report, the sample contained 21.37 mg of codeine per 5 ml dosage, instead of 10 mg specified on the label. Two other samples from different batches, however, showed normal codeine levels. Excessive consumption of cough syrup with high levels of codeine can lead to health implications such as sedation, behavioral changes and drug dependence.
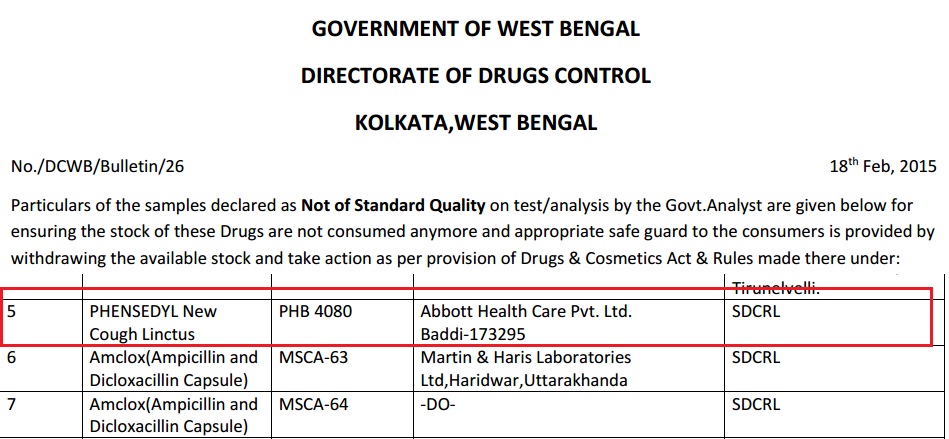
In February, West Bengal listed the potentially tainted batch as “not of standard quality” in a monthly publication. The bulletin, which is posted on the regulator’s website, is supposed to alert consumers and pharmacies in the state to suspect drugs. But the West Bengal drug controller, C. M. Ghosh, said he does not have the resources to follow it up.
Pointing Fingers
However, the ironic part is both states West Bengal and Himachal Pradesh who are involved in the allegations are not willing to take responsibility and are saying-

Sketchy Drug Regulation
India has nearly 1 in every 22 drug samples of sub-standard quality. In order to tackle this India is grossly under staffed with only 1,500 drug inspectors responsible for more than 10,000 factories, supplying medicines for a population of 1.2 billion and exporting to nearly 200 countries. The federal government wants to improve regulation of the key sector, and plans to spend $263 million in the next three years to strengthen the national and state regulatory system with additional equipment and staff and new laboratories.
Enjoyed reading this news story? For MORE medical discussions,image cases, MCQs and cases download the DAILY ROUNDS app for FREE! at App Store or Play Store
Original Post : Economic Times
Image Credits: W.Bengal Directorate of Drugs Control

