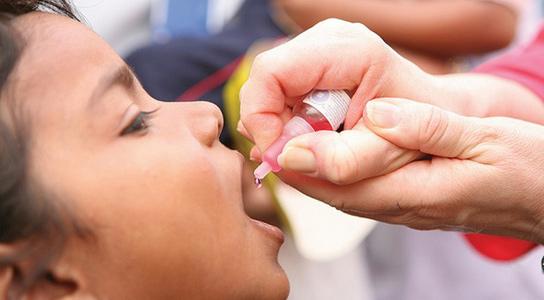
Why an Indian-made vaccine is seen as game-changer in fight against rotavirus
A vaccine made by Pune-based Serum Institute has shown an inspiring 66.7% efficacy against severe rotavirus gastroenteritis in infants in Niger(West Africa). The oral vaccine is now considered as a gamechanger in the global war against the virus. As per the WHO, the virus kills around 600 children every day, out of which 22% are in India.
Serum Institute, which is the world’s biggest vaccine make makes and sells over 1.3 billion doses every year around the world.
What’s special about the new vaccine?
Rotovac is a vaccine which became part of India’s Universal Immunisation Programme(UIP) when the vaccine was introduced in 4 states in April 2016. It was extended to 5 more states last month. The major progression with the new drug- which will be named Rotasiil, is that, it doesn’t need refrigeration, unlike Rotavac. This makes it easier to be stored in low-income nations where the virus poses the biggest threat to kids.
The Phase 3 trials have been completed in Niger and shows good efficacy in heat-challenging situations. “This trial brings a vaccine which is adapted to African settings to those who need it most,” AFP quoted Sheila Isanaka, assistant professor of nutrition at Harvard University and co-author of the study in the New England Journal of Medicine, as saying.
The Phase 3 trials
The trial included 3,508 infants of whom 1,780 would be in the vaccine group and 1,728 in the placebo group. It was done over the period from August 2014 to November 2015. At 28 days, after the third dose of the vaccine or placebo was administered, severe rotavirus gastroenteritis was reported in 31 infants in the vaccine group and 87 in the placebo group, putting the efficacy at 66.7%
In a previous trial in Kenya, Mali and Ghana, the RotaTeq vaccine’s efficacy was found to be 39.4. Similar trial in S.Africa and Malawi showed the efficacy of Rotarix was 61.2%. In the new trial, there was a higher incidence of severe disease than in the other two trials. During the trial, the vaccine was stored at 25 degrees Celsius and not refrigerated.
The vaccine is licensed in India. It’s currently under WHO pre-qualification process. Once it gets approved, low-income nations could procure the vaccine at an affordable price. The manufacturer hopes to roll it out in August-September of this year.
Image credits: thebetterindia.com
Images may be indicative